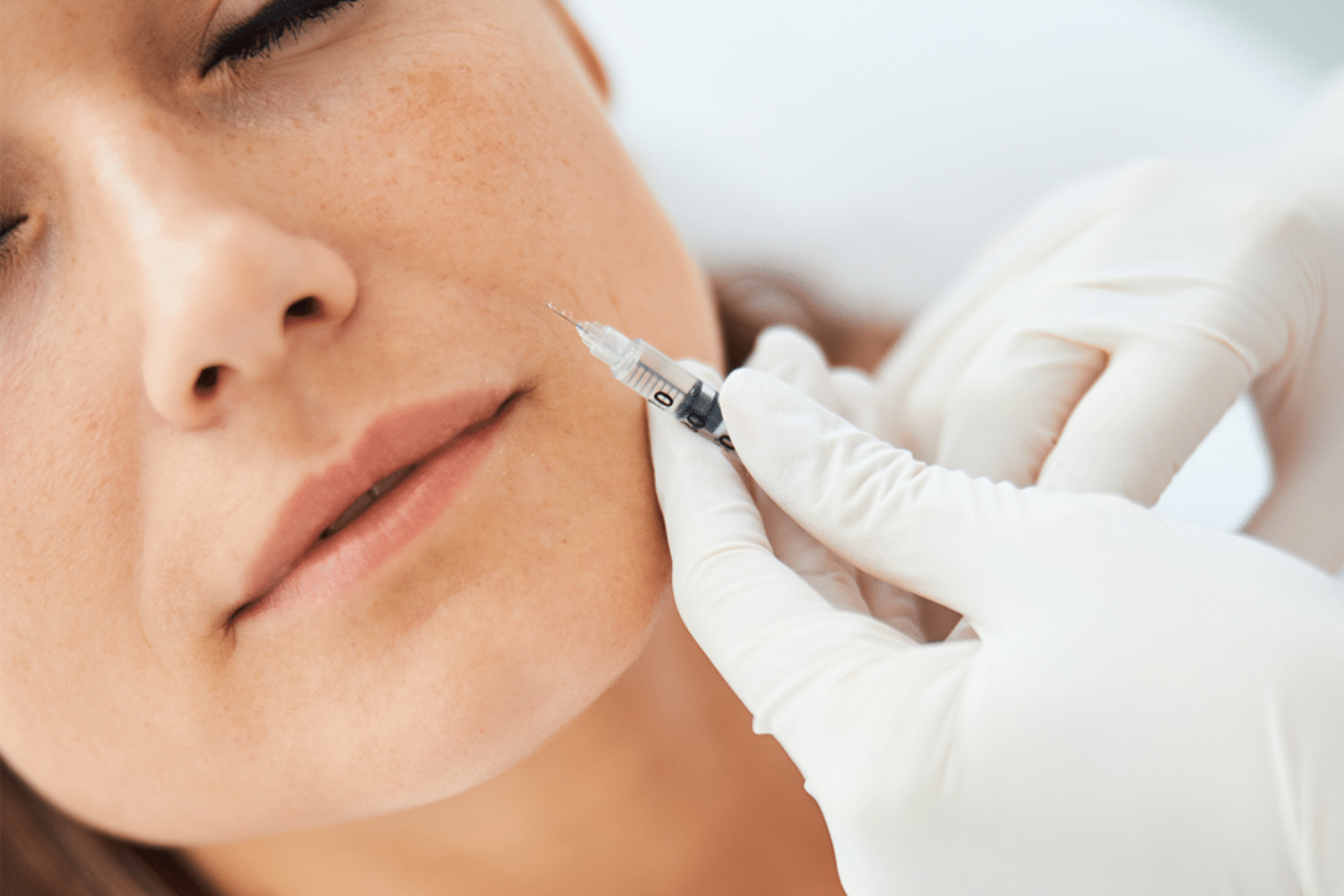
Next-generation flexible hyaluronic acid dermal fillers provide natural-looking results through XpresHAn Technology
Fort Worth, TX – (December 12, 2016) – Galderma, a global leader focused on medical solutions in skin health, announced today it has received U.S. Food and Drug Administration (FDA) approval of two new products for the treatment of nasolabial folds (NLF) or “laugh lines,” in patients over the age of 21.1,2 Restylane® Refyne was approved for the treatment of moderate to severe facial wrinkles and folds and Restylane® Defyne for the treatment of moderate to severe, deep facial wrinkles and folds.1,2 These scientifically-advanced gels are manufactured with XpresHAn Technology (pronounced ex-ˈspre-shən), creating gels that offer a range of flexibility and support 3,4,5 for varied patient needs. Restylane® Refyne and Restylane® Defyne have been shown to maintain effectiveness for the treatment of laugh lines for up to 12 months.1,2
“Even with current approved options, many of my patients are still looking for different solutions to treat their laugh lines,” said Boca Raton-based oculoplastic surgeon and Restylane® Refyne clinical investigator Steven Fagien, MD. “These new products are flexible and are designed to meet different patient needs. I am excited to offer these next-generation hyaluronic acid (HA) dermal fillers in my practice.”
“Restylane® Refyne and Restylane® Defyne are the latest FDA-approved advancements in HA dermal fillers and align with Galderma’s mission to help individuals achieve natural-looking results through treatments with a long-standing history of proven safety and efficacy,” said Kelly Huang, PhD – VP & General Manager of the U.S. Aesthetic and Corrective business of Galderma. “We saw an opportunity to address a common concern for patients who have not yet tried a dermal filler by designing gels that provide natural-looking results. With these new brands, the Restylane® family of products now represents the broadest offering of HA dermal fillers in the U.S.”
The FDA approval was based on two pivotal, double-blinded, randomized, active-controlled Phase 3 studies investigating Restylane® Refyne and Restylane® Defyne (involving 171 and 162 subjects, respectively) to evaluate their safety and effectiveness. In both studies, Restylane® Refyne and Restylane® Defyne met the studies’ endpoints, with both products showing a clinically meaningful improvement in wrinkle severity for up to 12 months in the majority of patients. Study investigators used the Wrinkle Severity Rating Scale (WSRS), a validated 5-point measure of the size and depth of the wrinkles, with grade 1 defined as absence of wrinkles and grade 5 as extremely deep and long wrinkles. Investigators reported that 79% of Restylane® Refyne subjects and 77% of Restylane® Defyne subjects had at least a 1-grade improvement on the WSRS after 6 weeks. Subjects also performed self-assessments (SSA) of wrinkle severity, with most subjects reporting at least a 1-grade improvement in SSA scores with Restylane® Refyne and with Restylane® Defyne after 6 weeks.1,2
“Many of my patients are interested to learn about the latest products that can help them achieve natural-looking results, but are oftentimes unsure about starting dermal fillers,” said San Diego-based board-certified dermatologist Mitch Goldman, MD. “The introduction of these next-generation HA dermal fillers with XpresHAn Technology has the potential to change my patients’ views on fillers. Restylane® Refyne and Restylane® Defyne provide options for patients who want to make sure they can achieve results that provide options for patients who want to make sure they can achieve natural-looking results which is a key need my patients express every day.”
After initial treatment, injection site responses (redness, swelling, bruising, lump/bump formation, pain/tenderness) were predominantly mild or moderate in intensity, temporary (typically with a duration of one to two weeks), and similar for the Restylane® products.
XpresHAn Technology
Restylane® Refyne and Restylane® Defyne were first approved in Europe in 2010 under the brand name Emervel® and have a proven safety profile demonstrated by more than one million treatments worldwide.7 Restylane® Refyne and Restylane® Defyne are dermal fillers injected under the (facial) skin. Once in place, they help smooth away your “laugh lines” – the wrinkles and folds that may form at the sides of your nose and run down toward the corners of your mouth. These next-generation dermal fillers were designed using a unique manufacturing process called XpresHAn Technology, which creates a smooth, injectable gel that can give your skin natural-looking results. XpresHAn Technology customizes the degree of HA crosslinking in each product, resulting in gels with a range of flexibility and support characteristics for different patient needs. Restylane® Refyne is designed to be very flexible and provide subtle support, while Restylane® Defyne is designed to be less flexible and provide additional support.
Phase 4 Studies
Galderma is conducting three ongoing Phase 4 clinical studies to investigate the effects of Restylane® Refyne and Restylane® Defyne as patients conduct everyday expressions and is looking forward to sharing these results as they become available.
About Restylane
With over 30 million treatments worldwide and counting,8 the Restylane® line of hyaluronic acid fillers is used to help smooth away wrinkles (Restylane® & Restylane® Lyft), create fuller and more accentuated lips (Restylane® Silk), and add lift and volume to the cheeks (Restylane® Lyft). All Restylane® products work to enhance facial features and give long-lasting, yet non-permanent results.
To learn more about the Restylane® line of products, visit www.RestylaneUSA.com.
About Galderma
Created in 1981, Galderma is now present in over 100 countries with an extensive product portfolio of medical solutions to treat a range of dermatological conditions. The company partners with health care professionals around the world to meet the skin health needs of people throughout their lifetime. Galderma is a leader in research and development of scientifically-defined and medically-proven solutions for the skin, hair and nails.
Strategic brands in the U.S. include Epiduo® Gel, Epiduo® Forte Gel, Oracea® Capsules, Clobex® Spray, Differin® Gel, Mirvaso® Gel, MetroGel® Gel, Soolantra® Cream, Vectical® Cream, Tri-Luma® Cream, Cetaphil®, Benzac® Acne Solutions, Restylane®, Restylane® Silk, Restylane® Lyft, Dysport® and Sculptra® Aesthetic.
For more information, please visit www.galdermausa.com and www.galderma.com.
All trademarks are the property of their respective owners.